How It Works
- "Crack" longer chain hydrocarbons into shorter chains that burn easier and cleaner
- Take straight-chain hydrocarbons and turn them into ring compounds that generate free hydrogen, allowing the mixture to burn more efficiently.
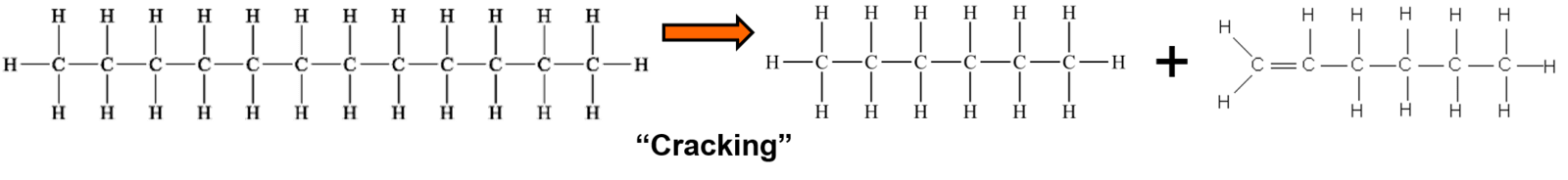
Some then convert to aromatic rings and release a bit of free hydrogen into the fuel mix. All this is from the catalysts stripping some hydrogen atoms off of the carbon atoms (see the catalysis page for more on how that works).
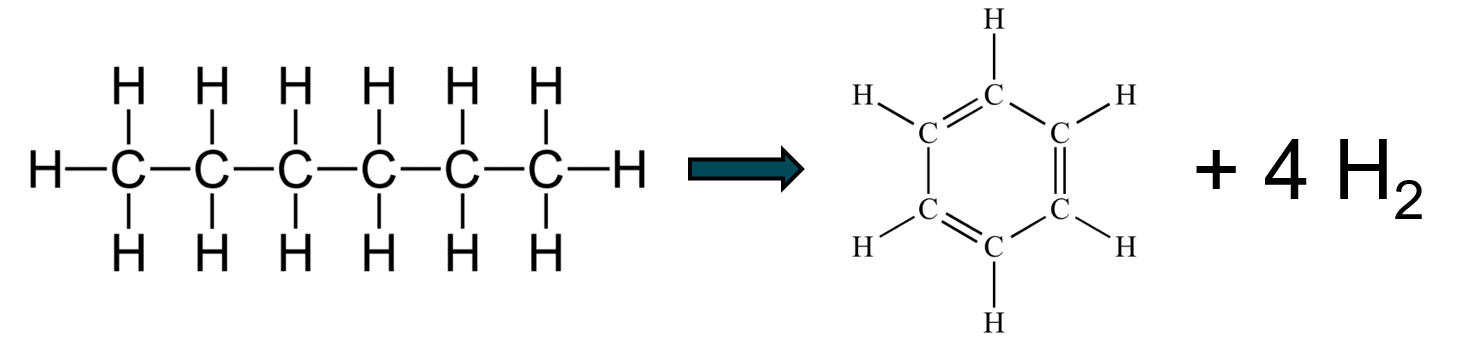
The hydrogen burns fast & hot to help break up the rest of the long chains into shorter chains that burn more efficiently and achieve a more complete combustion. Both of these (shorter molecules and free hydrogen) allow for a faster and cleaner burn in the combustion chamber, increasing the efficiency. It's not magic, just chemistry and physics.
Technical Overview of the Rentar Fuel Catalyst
Emissions Discussion
Emissions must also be considered when employing this technology, and fortunately the Rentar Fuel Catalyst improves emissions. The more efficient burn reduces the number of unburned hydrocarbons, and the improved fuel consumption will directly reduce the CO2 in the exhaust since all carbon in CO2 originated from the fuel source. NOx is heavily dependent on flame temperature; however, one must consider local variations in flame temperature due to local variations in fuel-to-air ratios within the combustion cylinder. Above a certain temperature, NOx increases exponentially with temperature, and local hot spots can generate relatively large amounts of NOx. Thus, atomization and mixing are very important in the design. From a global standpoint, the global pressure and temperature during combustion will determine the power output, and for the same global temperature and pressure, the power output will be the same regardless of the type of fuel used to generate the high temperature. Therefore, two engines with the same global flame temperature but different mixing can have similar power outputs with substantially different amounts of NOx emissions. Due to the effects on the fluid properties, mixing is likely improved as evident in the reduction in NOx emissions using this technology.
CO emissions are also reduced with the Rentar system. However, the cause for this reduction is likely a combination of multiple physical and chemical processes. Conditioned fuels coming out of the Rentar Fuel Catalyst are more energy-efficient for combustion and more likely to experience complete combustion, leading to less production of CO from incomplete combustion. From an equilibrium standpoint, the amount of CO, due to dissociation of CO2, increases with temperature. The improved mixing and reduction of local hotspots in the cylinder likely leads to a reduction in CO2 dissociation and improved combustion efficiency. From a chemical kinetic standpoint, the conversion of CO to CO2 is much faster at higher temperatures. Less mixing leads to both local hot and cold spots. Additionally, heat loss at the wall can affect the local temperature and CO formation. The improved mixing, as evident in the reduction of NOx emissions, locally reduces both CO2 dissociation in higher temperature regions and increases the conversion of CO to CO2 in lower temperature regions.
In summary, the Rentar Fuel Catalyst system advantageously affects the chemical composition and the properties of the fuel. Reduced fuel consumption improves fuel economy and reduces emissions. The improved mixing also aids to reduce NOx emissions and CO emissions. Due to the improved performance and emissions and the relative ease of adapting existing engine technologies for use with the Rentar Fuel Catalyst, the technology is very attractive to a wide variety of applications.
